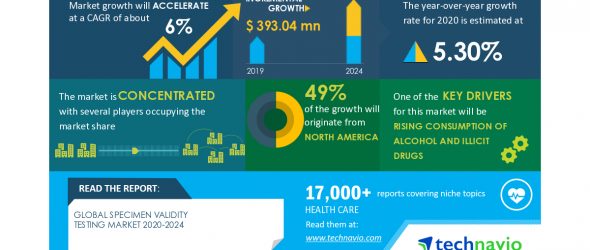
LONDON–(BUSINESS WIRE)–The global specimen validity testing market is expected to grow by USD 393.04 million as per Technavio. This marks a significant market slowdown compared to the 2019 growth estimates due to the impact of the COVID-19 pandemic in the first half of 2020. However, steady growth is expected to continue throughout the forecast period, and the market is expected to grow at a CAGR of about 6%.
Request challenges and opportunities that influence COVID-19 pandemic – Request free sample pages of the specimen validity testing market
Read the 120-page report with TOC on “Specimen Validity Testing Market Analysis Report by Type (Products and Services), Geography (North America, Europe, Asia, and ROW), and the Segment Forecasts, 2020-2024”.
https://www.technavio.com/report/global-specimen-validity-testing-market-industry-analysis
The market is driven by the growing consumption of alcohol and illicit drugs. In addition, the growing adoption of inorganic growth strategies by vendors is anticipated to boost the growth of the specimen validity testing market.
Globally, the consumption of alcohol and illicit drugs such as opioids, cocaine, amphetamines, and cannabis has significantly increased over the years. In 2017, about 6% of the population, aged between 15-64 years used illicit drugs across the globe. Every year, the consumption of drugs claims about 11-12 million lives worldwide. To counter the increasing incidences of drug addiction and drug abuse, several government, corporate, and law-enforcement agencies are adopting specimen validity testing services. In addition, many governments are also enforcing new regulations and initiating awareness campaigns to control drug abuse practices. These developments are increasing the demand for drug abuse testing and specimen validity testing products and services, leading to the growth of the market.
Buy 1 Technavio report and get the second for 50% off. Buy 2 Technavio reports and get the third for free.
View market snapshot before purchasing
Major Five Specimen Validity Testing Companies:
Abbott Laboratories
Abbott Laboratories operates its business through segments such as Established Pharmaceuticals, Nutritionals, Diagnostics, and Medical Devices. The company offers Alere iCup drug screen. It minimizes urine exposure and tests for up to 13 drugs in one device and offers specimen validity combinations, such as creatinine, glutaraldehyde, nitrite, oxidants/bleach, pH, and specific gravity adulteration.
Alfa Scientific Designs Inc.
Alfa Scientific Designs Inc. operates its business through segments such as Products, Manufacturing Services, and Development Services. Instant-view Push Button Multi-Drug Of Abuse Urine Cup is the key offering of the company. It has a split specimen feature, which allows the operator to initiate the test when a patient is ready to read the results. Also, its flat test windows enable operators to photocopy the results for a permanent patient record.
American Bio Medica Corp.
American Bio Medica Corp. operates its business through a unified business segment. The company offers Rapid TOX Cup II. It is available with an integrated Specimen Validity Test (SVT) strip, which can be located to the left of the Rapid TOX Cup II logo printed on the cup.
Laboratory Corporation of America Holdings
Laboratory Corporation of America Holdings operates its business through segments such as LabCorp Diagnostics and Covance Drug Development. The company offers urine drug testing services through its Substance Abuse and Mental Health Services Administration (SAMHSA)1-certified laboratories. It offers specimen validity testing to detect adulterants or specimen substitution resulting from a donor’s attempts to mask drug use.
Premier Biotech Inc.
Premier Biotech Inc. operates its business through segments such as Products and Lab Services. The company offers products such as Premier Bio-Cup, Premier Bio-Dip, and U-Tox. These products feature built-in specimen validity tests.
Register for a free trial today and gain instant access to 17,000+ market research reports.
Technavio’s SUBSCRIPTION platform
Specimen Validity Testing Market Type Outlook (Revenue, USD Million, 2020-2024)
Specimen Validity Testing Market Regional Outlook (Revenue, USD Million, 2020-2024)
- North America
- Europe
- Asia
- ROW
Technavio’s sample reports are free of charge and contain multiple sections of the report, such as the market size and forecast, drivers, challenges, trends, and more.
Related Reports on Healthcare Include:
Global Drugs of Abuse Testing Market – Global drugs of abuse testing market by product (instruments and consumables) and geography (Asia, Europe, North America, and ROW).
Global In-vitro Toxicity Testing Market – Global in-vitro toxicity testing market by end-users (pharmaceutical and biotechnology companies, academic and research institutions, and others) and geography (Asia, Europe, North America, and ROW).
About Technavio
Technavio is a leading global technology research and advisory company. Their research and analysis focus on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions.
With over 500 specialized analysts, Technavio’s report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio’s comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.